Abstract
Introduction: Aplastic anemia is thought to occur most often because of an aberrant autoreactive cytotoxic T-cell response that destroys hematopoietic stem cells. Therefore, treatment involves either immunosuppressive therapy utilizing antithymocyte globulin and a calcineurin inhibitor, or bone marrow transplantation. General guidelines recommend stopping calcineurin therapy after 4-6 months. However, anecdotal delayed responses in patients treated with extended calcineurin inhibition (ECI), combined with the principle that solid organ transplant recipients routinely receive calcineurin inhibitor therapy for years, prompted this retrospective analysis of ECI. Such information will be particularly relevant to patients who do not have a matched sibling bone marrow donor, have relative contraindications to a second course of intensive immunosuppressive therapy, or are not able to access a promising investigational protocol.
Objective: (1) To compare peripheral blood count and survival outcomes of severe aplastic anemia patients treated with extended calcineurin inhibitor therapy to patients treated with matched sibling-donor bone marrow transplantation (MRD-BMT). (2) To compare peripheral blood count improvement from 6 month values in the ECI cohort to quantify the effect of extending therapy.
Design, Setting, and Patients: Patients were selected from a database of fifty-three patients with severe and very severe aplastic anemia treated between 1994 and 2017 at the Indiana University Melvin and Bren Simon Cancer Center. Treatment groups included extended calcineurin inhibitor (defined as treatment greater than six months; n=15) and matched sibling-donor bone marrow transplantation (n=9). Patients who received 6 months or less of calcineurin inhibition, matched unrelated transplantation, other second-line therapies, supportive care, or those with missing data were excluded from analysis.
Methods: Patients in the ECI arm were initially treated with anti-thymocyte globulin, glucocorticoids, and calcineurin inhibitor (cyclosporine or tacrolimus) followed by continuous calcineurin inhibition for longer than 6 months. Immediate pre-treatment counts were those obtained at the time of admission for therapy. White blood cell (WBC), hemoglobin (Hb), and platelet (Plt) counts were monitored retrospectively and improvement from the pre-treatment values was analyzed using the Mann-Whitney nonparametric U test. The influence of transfusions was accounted for by excluding counts obtained within one week of transfusion.
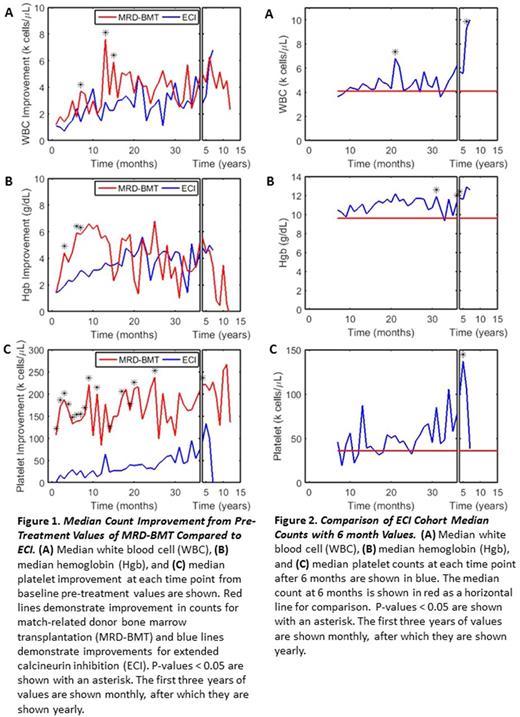
Results: Median follow-up time was 34mo and 54mo respectively for the ECI and BMT cohorts. Figure 1 compares median improvement in cell counts between the ECI and MRD-BMT treatment arms. Figure 2 compares median cell counts of the ECI cohort after 6 months compared to their 6 month values. Patients with severe aplastic anemia who received MRD-BMT had a statistically significant greater improvement in median WBC counts from their pre-treatment values for up to 15 months, hemoglobin for up to 7 months, and platelet counts for up to 48 months compared to those who received ECI. However, beyond these times the difference in outcomes was not significant. Survival for ECI group was 88% versus 89% for patients undergoing BMT, p-value 0.749. Patients with severe aplastic anemia who were treated with calcineurin inhibition beyond 6 months had a statistically significant improvement in their hemoglobin beginning at 30 months and an otherwise general non-significant improvement in their counts.
Conclusion: In this population of patients with severe aplastic anemia, MRD-BMT demonstrated greater improvement of peripheral counts compared to ECI initially, but the difference eventually became consistently not significant. Patients who received extended calcineurin inhibition demonstrated a consistent but non-significant improvement in their counts, except for perhaps hemoglobin, when compared to their 6 month values.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal